Learning How To Learn Amino Acids doesn’t have to be daunting. This comprehensive guide, crafted by educational experts at LEARNS.EDU.VN, provides effective strategies, memory aids, and a deep understanding of amino acid properties. By mastering these building blocks of proteins, you can unlock a deeper understanding of biochemistry. Enhance your comprehension of amino acid classification, structural properties, and pH effects with our proven learning techniques.
1. What Are Amino Acids And Why Is Learning Them Important?
Amino acids are the fundamental building blocks of proteins, essential for all life processes. Learning them is important because they are the key to understanding protein structure, function, and interactions. A strong grasp of amino acids is foundational for success in biochemistry, molecular biology, medicine, and related fields. At LEARNS.EDU.VN, we provide the resources and strategies you need to master this crucial topic.
Amino acids (AAs) are organic compounds containing amine (-NH2) and carboxyl (-COOH) functional groups, along with a side chain (R group) specific to each amino acid. These side chains dictate the unique properties of each amino acid, influencing how proteins fold and interact.
Proteins, composed of chains of amino acids linked by peptide bonds, perform a vast array of functions in living organisms:
- Enzymes: Catalyze biochemical reactions.
- Structural components: Provide support and shape to cells and tissues (e.g., collagen, keratin).
- Hormones: Chemical messengers that regulate physiological processes (e.g., insulin).
- Antibodies: Part of the immune system, defending against foreign invaders.
- Transport molecules: Carry substances within the body (e.g., hemoglobin).
Understanding amino acids is critical because:
- Protein Structure: The sequence of amino acids determines a protein’s three-dimensional structure, which is essential for its function.
- Protein Function: The unique properties of each amino acid side chain influence how a protein interacts with other molecules and performs its specific role.
- Metabolic Pathways: Amino acids are involved in numerous metabolic pathways, including synthesis of other essential biomolecules.
- Genetic Diseases: Many genetic diseases result from mutations that alter amino acid sequences in proteins, leading to dysfunction.
Consider phenylketonuria (PKU), a genetic disorder where the body can’t process phenylalanine. This buildup can lead to serious health problems, highlighting the importance of understanding amino acid metabolism. According to the National Institutes of Health, early detection and dietary management can prevent severe complications in individuals with PKU.
LEARNS.EDU.VN provides comprehensive resources to help you:
- Memorize amino acid structures, names, and abbreviations.
- Understand the physicochemical properties of amino acids.
- Learn how amino acids influence protein structure and function.
- Explore the role of amino acids in metabolic pathways and diseases.
2. What Are The Most Effective Strategies To Memorize Amino Acids?
Effective memorization strategies for amino acids include flashcards, mnemonic devices, and repeated exposure. Utilizing visual aids, such as diagrams and molecular models, can also enhance retention. For a structured approach, LEARNS.EDU.VN offers tailored learning plans and interactive quizzes.
Memorizing the 20 common amino acids, their structures, names, abbreviations, and properties can be a significant challenge. Here are some of the most effective strategies:
2.1 Flashcards:
- Create flashcards with the amino acid name and structure on one side, and its three-letter code, one-letter code, and key properties on the other.
- Use spaced repetition: Review flashcards frequently at first, then gradually increase the intervals between reviews. Apps like Anki are excellent for this.
- Categorize flashcards by amino acid type (e.g., hydrophobic, polar, acidic, basic) to reinforce understanding of their properties.
2.2 Mnemonic Devices:
- Develop memorable phrases or acronyms to associate with each amino acid. For example:
- “A Very Large Iguana Leaps Merrily Past Wild Turkeys.” (Alanine, Valine, Leucine, Isoleucine, Methionine, Proline, Tryptophan).
- “His Lys Arg” (Histidine, Lysine, Arginine) – the positively charged (basic) amino acids.
- “Asp Glu” (Aspartate, Glutamate) – the negatively charged (acidic) amino acids.
2.3 Visual Aids:
- Use diagrams, molecular models, and online resources to visualize amino acid structures.
- Draw the structures yourself repeatedly to reinforce your memory.
- Color-code different parts of the amino acid structures (e.g., amine group, carboxyl group, side chain) to help you distinguish them.
2.4 Repetitive Memorization:
- Write out the names, structures, and abbreviations of the amino acids multiple times.
- Recite the information aloud to engage auditory memory.
- Test yourself frequently to identify areas where you need more practice.
2.5 Active Recall:
- Instead of passively reviewing information, actively try to recall it from memory.
- Use practice questions and quizzes to test your knowledge.
- Explain the concepts to someone else; teaching is a great way to reinforce learning.
2.6 Association:
- Associate each amino acid with a memorable image, story, or personal connection. For example:
- Glycine: “Gly” sounds like “glide,” so imagine a smooth, simple molecule gliding effortlessly.
- Tryptophan: “Trp” sounds like “trip,” so imagine feeling sleepy after eating turkey due to its tryptophan content.
2.7 Utilize Online Resources:
- Explore interactive tools and resources available on LEARNS.EDU.VN, such as:
- Amino acid quizzes and practice tests.
- Detailed explanations of amino acid properties and functions.
- Downloadable charts and diagrams.
2.8 Contextual Learning:
- Learn amino acids in the context of protein structure and function.
- Understand how the properties of amino acids contribute to protein folding, stability, and interactions.
- Apply your knowledge to real-world examples, such as enzyme mechanisms and protein-related diseases.
According to a study published in the journal “Medical Education,” spaced repetition and active recall are highly effective strategies for long-term retention of complex information. By incorporating these techniques into your study routine, you can significantly improve your ability to memorize and understand amino acids.
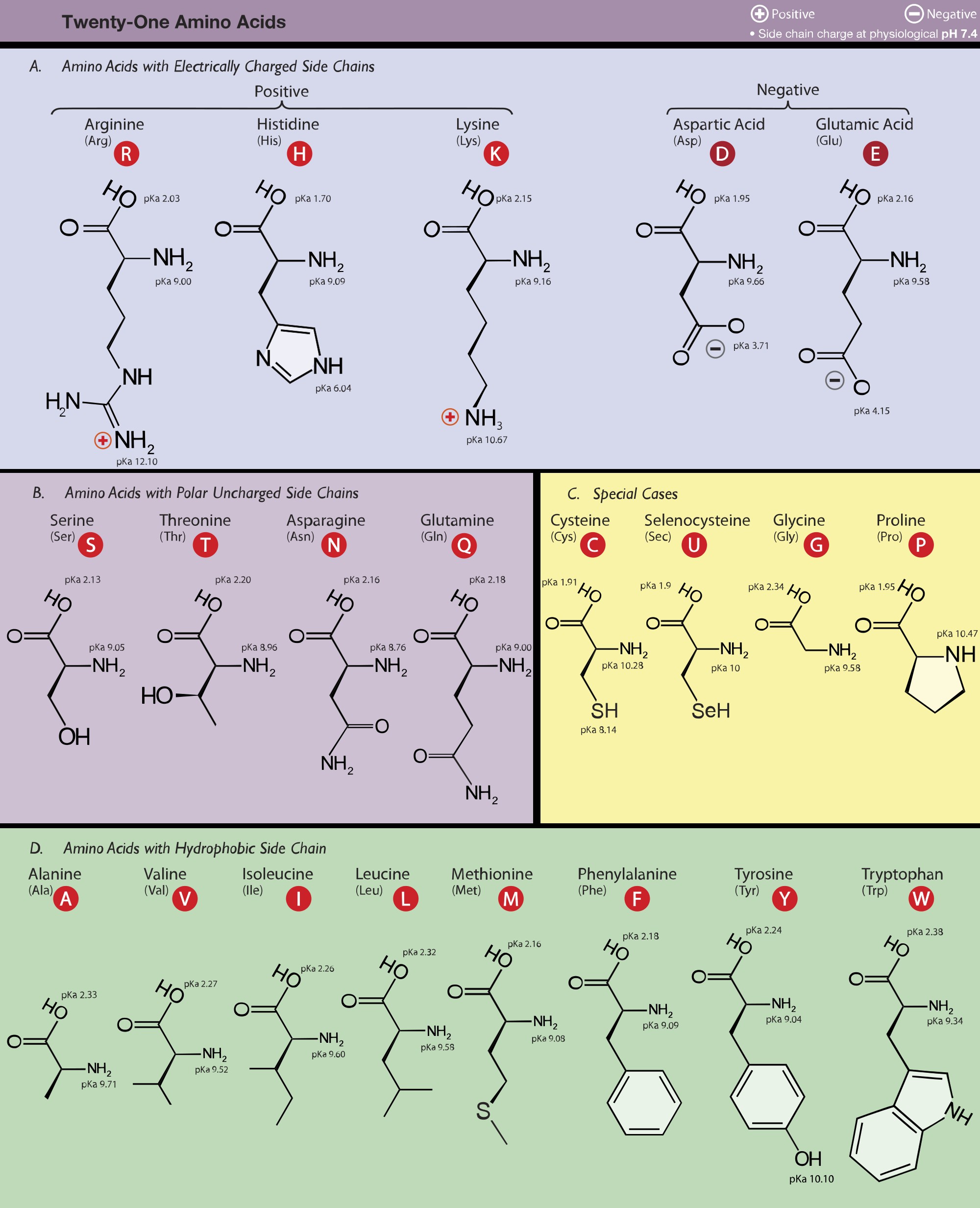
Figure 1. Table of amino acids. Image Source: Wikimedia
3. How Can You Understand The Physicochemical Properties Of Amino Acids?
To understand the physicochemical properties of amino acids, focus on their side chains (R groups). Group them based on polarity, charge, and size. Grasp how these properties influence protein folding and interactions. LEARNS.EDU.VN provides interactive tools to visualize these properties and their effects.
Understanding the physicochemical properties of amino acids is crucial for comprehending how they influence protein structure, function, and interactions. Here’s a breakdown of key properties and how to learn them effectively:
3.1 Classification by Side Chain:
-
Nonpolar, Hydrophobic Amino Acids: These amino acids have side chains that are primarily composed of hydrocarbons. They tend to cluster together in the interior of proteins, away from water. Examples include:
- Alanine (Ala, A)
- Valine (Val, V)
- Leucine (Leu, L)
- Isoleucine (Ile, I)
- Methionine (Met, M)
- Phenylalanine (Phe, F)
- Tryptophan (Trp, W)
- Proline (Pro, P)
-
Polar, Hydrophilic Amino Acids: These amino acids have side chains that contain polar groups (e.g., hydroxyl, amide, sulfhydryl). They tend to be located on the surface of proteins, interacting with water. Examples include:
- Serine (Ser, S)
- Threonine (Thr, T)
- Cysteine (Cys, C)
- Tyrosine (Tyr, Y)
- Asparagine (Asn, N)
- Glutamine (Gln, Q)
-
Acidic (Negatively Charged) Amino Acids: These amino acids have side chains that contain a carboxyl group, which is negatively charged at physiological pH. Examples include:
- Aspartate (Asp, D)
- Glutamate (Glu, E)
-
Basic (Positively Charged) Amino Acids: These amino acids have side chains that contain an amine group, which is positively charged at physiological pH. Examples include:
- Lysine (Lys, K)
- Arginine (Arg, R)
- Histidine (His, H)
3.2 Key Physicochemical Properties:
- Hydrophobicity: The tendency of an amino acid to repel water. Hydrophobic amino acids tend to cluster together in the interior of proteins.
- Polarity: The tendency of an amino acid to interact with water. Polar amino acids tend to be located on the surface of proteins.
- Charge: The electrical charge of an amino acid at a given pH. Acidic amino acids are negatively charged, basic amino acids are positively charged, and neutral amino acids have no charge.
- Size: The physical size of an amino acid. Larger amino acids can create steric hindrance, affecting protein folding and interactions.
- Hydrogen Bonding: The ability of an amino acid to form hydrogen bonds with other molecules. Polar amino acids are good hydrogen bond donors and acceptors.
- Aromaticity: The presence of an aromatic ring in the side chain. Aromatic amino acids can absorb UV light and participate in pi-stacking interactions.
3.3 Understanding the Impact on Protein Structure and Function:
- Protein Folding: The hydrophobic effect (tendency of hydrophobic amino acids to cluster together) is a major driving force in protein folding.
- Protein Stability: Hydrogen bonds, salt bridges (ionic interactions), and disulfide bonds contribute to protein stability.
- Protein-Protein Interactions: The properties of amino acids on the surface of proteins determine how they interact with other proteins.
- Enzyme Catalysis: The side chains of amino acids in the active site of an enzyme determine its substrate specificity and catalytic mechanism.
3.4 Strategies for Learning:
- Create Charts and Tables: Organize amino acids by their properties and create charts summarizing their key characteristics.
- Use Molecular Visualization Software: Programs like PyMOL and VMD allow you to visualize amino acid structures and their interactions in proteins.
- Relate Properties to Function: Understand how the properties of each amino acid contribute to the function of specific proteins. For example, the hydroxyl group of serine is essential for the activity of many enzymes.
- Practice Problems: Solve problems that require you to predict how amino acid properties will affect protein structure and function.
- Utilize LEARNS.EDU.VN Resources:
- Interactive quizzes and practice tests to assess your understanding.
- Detailed explanations of amino acid properties and their impact on protein structure.
- Case studies illustrating the role of amino acids in enzyme mechanisms and protein-related diseases.
According to a study published in the journal “Biochemistry Education,” students who actively engage with the material and relate it to real-world examples demonstrate a deeper understanding of amino acid properties. By using these strategies and resources, you can develop a strong foundation in this essential topic.
4. How Do pH Levels Affect Amino Acids And Their Charges?
pH levels significantly affect amino acids by influencing the protonation state of their amine and carboxyl groups, as well as ionizable side chains. At low pH, amino acids tend to be positively charged; at high pH, they become negatively charged. Understanding this is crucial for predicting protein behavior. LEARNS.EDU.VN offers interactive simulations to explore these effects.
The pH of a solution significantly affects the charge of amino acids due to the presence of ionizable groups (amine, carboxyl, and certain side chains). Here’s how pH levels impact amino acids and their charges:
4.1 Understanding pH and pKa:
- pH: A measure of the acidity or basicity of a solution. Lower pH indicates higher acidity (excess of H+ ions), while higher pH indicates higher basicity (excess of OH- ions).
- pKa: A measure of the acidity of a specific group in a molecule. It represents the pH at which half of the molecules in a solution are protonated and half are deprotonated.
4.2 Ionizable Groups in Amino Acids:
- Amino Group (-NH2): Can accept a proton (H+) to become positively charged (-NH3+). The pKa of the amino group is typically around 9-10.
- Carboxyl Group (-COOH): Can donate a proton (H+) to become negatively charged (-COO-). The pKa of the carboxyl group is typically around 2-3.
- Side Chains: Some amino acids have ionizable side chains with their own pKa values. Examples include:
- Aspartate (Asp, D): Carboxyl group (pKa ≈ 3.7)
- Glutamate (Glu, E): Carboxyl group (pKa ≈ 4.1)
- Histidine (His, H): Imidazole ring (pKa ≈ 6.0)
- Cysteine (Cys, C): Thiol group (pKa ≈ 8.3)
- Tyrosine (Tyr, Y): Hydroxyl group (pKa ≈ 10.1)
- Lysine (Lys, K): Amino group (pKa ≈ 10.5)
- Arginine (Arg, R): Guanidino group (pKa ≈ 12.5)
4.3 Effect of pH on Amino Acid Charge:
-
Low pH (Acidic Conditions): In a solution with a pH much lower than the pKa values of the amino and carboxyl groups, both groups will be protonated. The amino acid will have a positive charge.
- -NH2 + H+ → -NH3+ (protonated, positive charge)
- -COOH (protonated, neutral charge)
- Net charge: +1
-
Neutral pH (Physiological Conditions): At a pH around 7, the carboxyl group will be deprotonated and negatively charged, while the amino group will be protonated and positively charged. The amino acid will exist as a zwitterion (a molecule with both positive and negative charges, but a net charge of zero).
- -NH2 + H+ → -NH3+ (protonated, positive charge)
- -COOH → -COO- + H+ (deprotonated, negative charge)
- Net charge: 0
-
High pH (Basic Conditions): In a solution with a pH much higher than the pKa values of the amino and carboxyl groups, both groups will be deprotonated. The amino acid will have a negative charge.
- -NH3+ → -NH2 + H+ (deprotonated, neutral charge)
- -COOH → -COO- + H+ (deprotonated, negative charge)
- Net charge: -1
4.4 Isoelectric Point (pI):
- The isoelectric point (pI) is the pH at which an amino acid or protein has a net charge of zero.
- For amino acids with non-ionizable side chains, the pI is the average of the pKa values of the amino and carboxyl groups.
- pI = (pKa1 + pKa2) / 2
- For amino acids with ionizable side chains, the pI is calculated by averaging the pKa values of the two groups whose protonation states change around neutrality.
4.5 Importance of Understanding pH Effects:
- Protein Structure and Function: The charge of amino acids affects protein folding, stability, and interactions with other molecules.
- Enzyme Catalysis: The ionization state of amino acid side chains in the active site of an enzyme can affect its catalytic activity.
- Protein Purification: Techniques like ion exchange chromatography rely on the charge of proteins to separate them.
- Biological Processes: pH changes in the body can affect protein function and overall health.
4.6 Strategies for Learning:
- Draw Titration Curves: Plot the charge of an amino acid as a function of pH to visualize how the protonation state changes.
- Calculate pI Values: Practice calculating the isoelectric point of different amino acids.
- Relate pH Effects to Protein Behavior: Understand how pH changes can affect protein structure, function, and interactions.
- Use Online Resources:
- Interactive simulations on LEARNS.EDU.VN to explore the effects of pH on amino acid charge.
- Practice problems and quizzes to test your understanding.
- Case studies illustrating the role of pH in biological processes.
According to a study published in the journal “Biophysical Chemistry,” the charge state of amino acids is a critical determinant of protein structure and function. By understanding how pH affects amino acid charge, you can gain a deeper appreciation for the complex interplay of factors that govern protein behavior.
5. How Do Amino Acids Form Peptide Bonds And Create Proteins?
Amino acids form peptide bonds through a dehydration reaction, linking the carboxyl group of one amino acid to the amine group of another. These peptide bonds link amino acids into polypeptide chains, which then fold into functional proteins. LEARNS.EDU.VN offers visual aids and animations to illustrate this process.
Amino acids are linked together to form peptides and proteins through a process called peptide bond formation. Here’s a detailed explanation of how this occurs:
5.1 Peptide Bond Formation:
- A peptide bond is a covalent bond that forms between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid.
- The formation of a peptide bond involves a dehydration reaction, where a molecule of water (H2O) is removed.
- The carboxyl group loses a hydroxyl group (-OH), and the amino group loses a hydrogen atom (-H). These combine to form water (H2O).
- The carbon atom from the carboxyl group then forms a covalent bond with the nitrogen atom from the amino group, creating the peptide bond (-CO-NH-).
5.2 Process:
- Alignment: Two amino acids align themselves such that the carboxyl group of one amino acid is adjacent to the amino group of the other.
- Dehydration: An enzyme (in the ribosome during protein synthesis) catalyzes the removal of a water molecule from the carboxyl and amino groups.
- Bond Formation: A covalent bond forms between the carbon atom of the carboxyl group and the nitrogen atom of the amino group.
- Peptide Bond: The resulting -CO-NH- linkage is the peptide bond.
5.3 Formation of Polypeptides and Proteins:
- When multiple amino acids are linked together by peptide bonds, they form a chain called a polypeptide.
- The sequence of amino acids in the polypeptide chain is determined by the genetic code in DNA.
- Polypeptides can range in length from a few amino acids to thousands.
- Proteins are complex biomolecules that consist of one or more polypeptide chains folded into a specific three-dimensional structure.
- The three-dimensional structure of a protein is essential for its function.
5.4 Key Concepts:
- N-terminus: The amino end of a polypeptide chain, which has a free amino group (-NH2).
- C-terminus: The carboxyl end of a polypeptide chain, which has a free carboxyl group (-COOH).
- Residue: An amino acid that is part of a polypeptide chain.
- Primary Structure: The sequence of amino acids in a polypeptide chain.
- Secondary Structure: Local folding patterns within a polypeptide chain, such as alpha-helices and beta-sheets.
- Tertiary Structure: The overall three-dimensional structure of a single polypeptide chain.
- Quaternary Structure: The arrangement of multiple polypeptide chains in a multi-subunit protein.
5.5 Factors Influencing Protein Structure:
- Amino Acid Sequence: The sequence of amino acids in a polypeptide chain determines its folding pattern and overall structure.
- Hydrophobic Effect: Hydrophobic amino acids tend to cluster together in the interior of the protein, away from water.
- Hydrogen Bonds: Hydrogen bonds between amino acid side chains stabilize protein structure.
- Salt Bridges: Ionic interactions between positively and negatively charged amino acid side chains stabilize protein structure.
- Disulfide Bonds: Covalent bonds between cysteine residues stabilize protein structure.
5.6 Importance of Understanding Peptide Bond Formation:
- Protein Synthesis: Understanding how peptide bonds form is essential for understanding how proteins are synthesized in the cell.
- Protein Structure and Function: The sequence of amino acids and the way they are linked together determine the structure and function of proteins.
- Drug Design: Many drugs target proteins, and understanding protein structure and function is essential for designing effective drugs.
- Biotechnology: Proteins are used in a variety of biotechnological applications, and understanding protein structure and function is essential for developing new technologies.
5.7 Strategies for Learning:
- Draw Diagrams: Draw diagrams illustrating the formation of a peptide bond between two amino acids.
- Use Molecular Models: Use molecular models to visualize the three-dimensional structure of proteins.
- Relate Structure to Function: Understand how the structure of a protein determines its function.
- Practice Problems: Solve problems that require you to predict how changes in amino acid sequence will affect protein structure and function.
- Utilize LEARNS.EDU.VN Resources:
- Animations illustrating the formation of peptide bonds and the folding of proteins.
- Interactive quizzes and practice tests to assess your understanding.
- Case studies illustrating the role of proteins in biological processes and diseases.
According to research published in “Nature Structural & Molecular Biology,” the precise arrangement of amino acids dictates the final conformation of a protein, which directly influences its biological activity. By studying these processes on LEARNS.EDU.VN, you can gain a comprehensive understanding of how proteins are made and how they work.
Figure 3. Amino acids joined by peptide bonds in blue. Image Source: Wikimedia Commons
6. What Are Some Common Mistakes Students Make When Learning Amino Acids And How To Avoid Them?
Common mistakes include rote memorization without understanding properties, neglecting pH effects, and not connecting amino acids to protein structure. Avoid these by focusing on conceptual understanding, using visual aids, and practicing problem-solving. LEARNS.EDU.VN provides targeted exercises to address these common pitfalls.
Learning amino acids can be challenging, and students often make common mistakes that hinder their understanding. Here are some of these mistakes and how to avoid them:
6.1 Rote Memorization Without Understanding Properties:
- Mistake: Memorizing amino acid names, structures, and abbreviations without understanding their physicochemical properties (hydrophobicity, charge, size, etc.).
- Consequence: Inability to predict how amino acids will behave in different environments or how they contribute to protein structure and function.
- Solution: Focus on understanding the properties of each amino acid and how they relate to their structure. Group amino acids based on their properties and learn the general characteristics of each group.
6.2 Neglecting pH Effects:
- Mistake: Ignoring the impact of pH on the charge of amino acids and their side chains.
- Consequence: Difficulty predicting how amino acids will behave at different pH levels and how this affects protein structure and function.
- Solution: Understand the concept of pKa and how it relates to the protonation state of amino acids. Practice drawing titration curves and calculating the isoelectric point (pI) of different amino acids.
6.3 Not Connecting Amino Acids to Protein Structure:
- Mistake: Learning amino acids in isolation without understanding how they contribute to the different levels of protein structure (primary, secondary, tertiary, and quaternary).
- Consequence: Inability to appreciate the role of amino acids in protein folding, stability, and interactions with other molecules.
- Solution: Learn how the properties of amino acids influence protein folding patterns, such as alpha-helices and beta-sheets. Understand how hydrophobic interactions, hydrogen bonds, salt bridges, and disulfide bonds contribute to protein stability.
6.4 Confusing Similar Structures:
- Mistake: Mixing up amino acids with similar structures, such as valine, leucine, and isoleucine.
- Consequence: Difficulty accurately identifying amino acids and predicting their properties.
- Solution: Pay close attention to the differences in the side chains of similar amino acids. Use visual aids and draw the structures repeatedly to reinforce your memory.
6.5 Overlooking Special Properties:
- Mistake: Failing to recognize the unique properties of certain amino acids, such as cysteine (formation of disulfide bonds), proline (disruption of alpha-helices), and glycine (flexibility).
- Consequence: Incomplete understanding of how these amino acids contribute to protein structure and function.
- Solution: Pay special attention to the unique properties of each amino acid and how they affect protein behavior.
6.6 Ignoring the Importance of the R-Group:
- Mistake: Not focusing enough on the R-group (side chain) of each amino acid. The R-group is what makes each of the 20 amino acids unique.
- Consequence: Difficulty differentiating between amino acids and understanding their individual properties.
- Solution: Spend the majority of your time focusing on the R-groups. Understand how the structure and properties of the R-group influence the overall behavior of the amino acid.
6.7 Relying Solely on Memorization:
- Mistake: Trying to memorize everything without understanding the underlying concepts.
- Consequence: Difficulty applying your knowledge to new situations or solving complex problems.
- Solution: Focus on understanding the principles and concepts behind amino acid properties and protein structure. Use memorization as a tool to reinforce your understanding, not as a substitute for it.
6.8 Not Practicing Enough:
- Mistake: Failing to practice problem-solving and apply your knowledge to real-world examples.
- Consequence: Inability to effectively use your knowledge in exams or in research settings.
- Solution: Solve practice problems, work through case studies, and apply your knowledge to real-world examples. Use online resources, such as those available on LEARNS.EDU.VN, to test your understanding and identify areas where you need more practice.
By avoiding these common mistakes and focusing on conceptual understanding, you can develop a strong foundation in amino acid chemistry and its applications in biochemistry and molecular biology.
7. How Do Uncommon Amino Acids Like Selenocysteine Differ From The Common Ones?
Uncommon amino acids, like selenocysteine, differ from the common 20 in their structure and how they are incorporated into proteins. Selenocysteine contains selenium instead of sulfur and is incorporated via a unique genetic code. LEARNS.EDU.VN provides detailed comparisons and explanations of these differences.
While there are 20 common amino acids that are universally found in proteins, there are also some uncommon or non-standard amino acids. These amino acids are not directly encoded by the genetic code but are incorporated into proteins through special mechanisms or post-translational modifications. Here’s a look at how uncommon amino acids differ from the common ones:
7.1 Selenocysteine (Sec, U):
- Structure: Selenocysteine is similar to cysteine, but it contains a selenium atom (Se) instead of a sulfur atom (S).
- Incorporation: Selenocysteine is incorporated into proteins during translation through a special mechanism involving a UGA codon (normally a stop codon) and a specific stem-loop structure in the mRNA called the SECIS element (selenocysteine insertion sequence).
- Function: Selenocysteine is found in a variety of enzymes, including glutathione peroxidases, thioredoxin reductases, and iodothyronine deiodinases. These enzymes play important roles in antioxidant defense, redox regulation, and thyroid hormone metabolism.
- Difference from Common Amino Acids: Selenocysteine is not directly encoded by the standard genetic code and requires a special mechanism for its incorporation into proteins.
7.2 Pyrrolysine (Pyl, O):
- Structure: Pyrrolysine is an amino acid with a unique side chain containing a pyrroline ring and a lysine moiety.
- Incorporation: Pyrrolysine is incorporated into proteins during translation in certain archaea and bacteria through a special mechanism involving a UAG codon (normally a stop codon) and a specific tRNA and tRNA synthetase.
- Function: Pyrrolysine is found in enzymes involved in methane metabolism, such as methyltransferases.
- Difference from Common Amino Acids: Pyrrolysine is not directly encoded by the standard genetic code and requires a special mechanism for its incorporation into proteins.
7.3 Post-Translationally Modified Amino Acids:
- Hydroxyproline and Hydroxylysine: These amino acids are formed by the hydroxylation of proline and lysine residues after they have been incorporated into a polypeptide chain.
- Phosphoserine, Phosphothreonine, and Phosphotyrosine: These amino acids are formed by the phosphorylation of serine, threonine, and tyrosine residues after they have been incorporated into a polypeptide chain.
- Formation: These modifications are catalyzed by specific enzymes and are important for regulating protein function and stability.
- Difference from Common Amino Acids: These amino acids are not directly encoded by the genetic code but are formed by post-translational modifications of common amino acids.
7.4 Key Differences Summarized:
| Feature | Common Amino Acids | Uncommon Amino Acids |
|---|---|---|
| Encoding | Directly encoded by genetic code | Requires special mechanisms |
| Incorporation | Standard translation process | Special tRNA, SECIS element |
| Examples | Alanine, Valine, Leucine, etc. | Selenocysteine, Pyrrolysine, etc. |
| Modification | No post-translational modification | Post-translational modification |
7.5 Importance of Understanding Uncommon Amino Acids:
- Expanding the Genetic Code: Uncommon amino acids expand the possibilities of protein structure and function beyond what is possible with the 20 common amino acids.
- Enzyme Function: Uncommon amino acids play important roles in the function of certain enzymes, such as those involved in antioxidant defense and methane metabolism.
- Biotechnology: Uncommon amino acids can be used to create proteins with novel properties for biotechnological applications.
7.6 Strategies for Learning:
- Focus on the Mechanism of Incorporation: Understand how uncommon amino acids are incorporated into proteins, including the role of special tRNAs and mRNA sequences.
- Learn the Function of Enzymes Containing Uncommon Amino Acids: Understand the role of these enzymes in biological processes.
- Compare and Contrast with Common Amino Acids: Understand how uncommon amino acids differ from common amino acids in terms of structure, properties, and function.
- Utilize LEARNS.EDU.VN Resources:
- Detailed explanations of uncommon amino acids and their incorporation mechanisms.
- Case studies illustrating the role of enzymes containing uncommon amino acids in biological processes.
By understanding the differences between common and uncommon amino acids, you can gain a more complete appreciation of the diversity and complexity of protein structure and function.
8. How Can Knowledge Of Amino Acids Be Applied To Real-World Situations, Like Medicine Or Biotechnology?
Knowledge of amino acids is crucial in medicine for understanding genetic diseases, drug design, and nutrition. In biotechnology, it’s vital for protein engineering and synthetic biology. LEARNS.EDU.VN provides case studies and examples of these applications.
Knowledge of amino acids is essential in various real-world situations, including medicine and biotechnology. Here are some specific examples of how amino acid knowledge is applied:
8.1 Medicine:
- Genetic Diseases: Many genetic diseases are caused by mutations in genes that encode proteins. These mutations can result in changes in the amino acid sequence of the protein, leading to a loss of function or altered function.
- Example: Phenylketonuria (PKU) is a genetic disorder caused by a deficiency in the enzyme phenylalanine hydroxylase, which is required to convert phenylalanine to tyrosine. Individuals with PKU must follow a special diet that is low in phenylalanine to prevent the buildup of this amino acid in the blood, which can lead to brain damage.
- Drug Design: Many drugs target proteins, and understanding the structure and function of these proteins is essential for designing effective drugs.
- Example: HIV protease inhibitors are drugs used to treat HIV infection. These drugs bind to the active site of the HIV protease enzyme, preventing it from cleaving viral proteins and inhibiting viral replication. The design of these inhibitors was based on knowledge of the amino acid sequence and structure of the HIV protease enzyme.
- Nutrition: Amino acids are essential nutrients that the body needs to build and repair tissues. Understanding the amino acid composition of foods is important for maintaining a healthy diet.
- Example: Essential amino acids are amino acids that the body cannot synthesize and must obtain from the diet. These include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. A diet that is deficient in one or more of these amino acids can lead to malnutrition and health problems.
8.2 Biotechnology:
- Protein Engineering: Protein engineering is the process of modifying the amino acid sequence of a protein to alter its properties or function.
- Example: Scientists have engineered enzymes with improved catalytic activity, stability, or substrate specificity for use in industrial processes. For example, the enzyme subtilisin has been engineered to be more resistant to oxidation and heat for use in laundry detergents.
- Synthetic Biology: Synthetic biology is the design and construction of new biological parts, devices, and systems. Amino acids are used as building blocks for creating synthetic proteins and peptides with novel functions.
- Example: Scientists have created synthetic proteins with unnatural amino acids that have unique properties, such as the ability to bind to specific molecules or catalyze novel reactions. These synthetic proteins can be used in a variety of applications, including drug delivery, biosensing, and materials science.
- Biopharmaceuticals: Many biopharmaceuticals, such as insulin and growth hormone, are proteins that are produced using recombinant DNA technology.
- Example: Insulin is produced in large quantities using genetically engineered bacteria or yeast. The human insulin gene is inserted into the bacteria or yeast, which then produce the protein. The insulin is then purified and used to treat diabetes.
8.3 Strategies for Learning:
- Study Case Studies: Explore case studies of diseases caused by mutations in amino acid sequences and how these diseases are treated.
- Research Drug Mechanisms: Investigate how drugs interact with proteins at the amino acid level.
- Analyze Protein Engineering Examples: Study examples of how protein engineering has been used to create proteins with improved properties.
- Utilize LEARNS.EDU.VN Resources:
- Case studies illustrating the role of amino acids in diseases and drug design.
- Examples of protein engineering and synthetic biology applications.
According to the Biotechnology Innovation Organization (BIO), advancements in understanding amino acids and protein structures have led to revolutionary treatments and therapies. By studying these applications on LEARNS.EDU.VN, you can see firsthand how your knowledge of amino acids can make a real difference in the world.
9. What Role Do Amino Acids Play In Enzyme Catalysis?
In enzyme catalysis, amino acids in the active site act as acids, bases, or nucleophiles, facilitating chemical reactions. Specific amino acids like histidine, serine, and cysteine are critical for enzyme function. learns.edu.vn provides detailed enzyme mechanism diagrams highlighting these roles.
Amino acids play critical roles in enzyme catalysis. Enzymes are biological catalysts that speed up chemical reactions in living organisms. The active site of an enzyme, where catalysis occurs, is formed by specific amino acid residues that participate directly in the reaction. Here are some key roles that amino acids play in enzyme catalysis:
**9.
