Introduction
State-Dependent Learning (SDL), a fascinating aspect of memory, describes the phenomenon where information learned in a specific state is most readily recalled when the individual returns to that same state. Imagine trying to remember something you learned while feeling anxious – you might find it easier to access that memory when you are anxious again. This concept, initially termed “dissociation of learning,” highlights how our internal and external environments during learning significantly impact memory retrieval.
The pioneering work of Girden and Culler in 1937 first illuminated SDL. They observed that dogs conditioned to flex their legs under the influence of curare, a muscle relaxant, only exhibited this learned response when redosed with curare. Conversely, learning in a normal state was not retrievable under curare, and vice versa. This groundbreaking experiment laid the foundation for understanding memory as not just a singular entity, but one that is intricately linked to the context of learning.
Since then, SDL has been observed across a diverse range of species, from invertebrates to humans, solidifying its fundamental nature in memory processes. Beyond pharmacological substances, various stimuli can induce state-dependent effects. These encompass electrical stimulation, hormonal fluctuations, mood variations, motivational drives, circadian rhythms, sleep stages, pain perception, and even environmental contexts. Considering this broad spectrum of influencing factors, it’s plausible that all memories possess some degree of state dependency, subtly shaped by our affective states, motivations, and interactions with our surroundings (Figure 1).
Figure 1. Inducing State Dependent Learning through Excitatory/Inhibitory Balance Modulation.
Alt Text: Diagram illustrating exogenous and endogenous stimuli inducing State Dependent Learning (SDL), and a passive avoidance paradigm example showing memory retrieval differences based on the presence or absence of SDL-inducing stimuli.
(A) Diverse Stimuli Inducing SDL: This panel showcases a range of factors known to trigger state-dependent learning, including drugs, electrical stimulation, hormones, mood, circadian rhythms, sleep, pain, and environmental context. These stimuli share the commonality of altering the internal “state” of the organism. (B) SDL in Passive Avoidance Paradigm: This section illustrates SDL using a passive avoidance task. Top: Memories formed under normal conditions are easily accessed in similar conditions. However, applying SDL-inducing stimuli before testing hinders retrieval. Bottom: Conversely, memories encoded under SDL-inducing stimuli are inaccessible under normal conditions but become retrievable when the same stimuli are reintroduced. The diagram highlights the interplay of excitation (E) and inhibition (I) in these processes.
Drug-based SDL studies have been particularly fruitful, revealing conditions and limitations of this phenomenon. Certain drugs, like phentobarbital, can induce complete dissociation, preventing memory transfer between drug and non-drug states. However, memory transfer can occur between drug-induced states that share pharmacological similarities. Interestingly, memory recovery in animal models has been observed during heightened arousal, through salient reminders, or after extensive overtraining. In humans, experimental cueing or prompting can also facilitate memory recovery in state-dependent scenarios.
While rodent models of reinforcement learning and passive avoidance tasks have extensively documented state-dependency with various psychoactive drugs, fear conditioning paradigms have, until recently, shown less susceptibility to these drug effects. The reasons behind these task-specific differences remain unclear, but potential explanations will be explored further in this discussion.
Decades of research, primarily between the 1960s and 1980s, provided a broad but somewhat shallow understanding of SDL, both in defining “state” and elucidating the underlying neurobiological mechanisms. “State” has been used broadly, encompassing brain conditions, mental states, or the overall condition of an individual. Fundamentally, it refers to alterations in the timing and routing of neuronal firing within specific brain networks. These shifts can modify the processing of stimulus features during encoding and potentially affect the function of neuronal comparators (involved in matching sensory inputs with stored information) during retrieval. The potential mechanisms of SDL are vast, as state-dependency is inherent to all levels of neuronal activity – from molecular and cellular processes to circuit dynamics, global network activity, and even consciousness itself. Therefore, factors defining neuronal states are likely to be found across these levels. Sleep serves as a compelling example. It’s an altered state of information processing characterized by distinct changes in neurotransmitter balance, activity redistribution in brain circuits, and slow oscillatory rhythms. Applying similar multi-level analyses to SDL is crucial for identifying the defining features of brain states that govern long-term memory encoding and retrieval.
Molecular Mechanisms of State Dependent Learning
In typical waking states, memory formation and retrieval are predominantly driven by excitatory neurotransmission, particularly involving N-methyl-D-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). The balance between excitation and inhibition generally leans towards excitation. However, shifts in this delicate equilibrium in either direction can underpin SDL. For instance, cholinergic mechanisms of SDL involve both reducing cholinergic function with scopolamine and enhancing it with physostigmine. Psychostimulants like amphetamine, meprobamate, cocaine, and caffeine are also frequently associated with SDL in both humans and rodents. Opiates, particularly morphine acting on µ-opioid receptors, also effectively induce state-dependent effects.
Nevertheless, the most substantial evidence for SDL points towards the activation of GABAergic neurotransmission and a consequent shift towards inhibition in the excitatory/inhibitory balance. The ionotropic GABAA receptor, a pentameric complex, plays a pivotal role. Many drugs interact with GABAA receptors, modulating their chloride ion conductance and thus influencing neuronal inhibition. However, drug-specific effects arise from their binding to distinct sites on the receptor complex. In rodents, various GABAA receptor agonists and positive allosteric modulators, including barbiturates, have been shown to induce SDL. Conversely, GABAB receptor agonists like baclofen are ineffective, supporting the notion that SDL is primarily mediated by GABAA receptors. Similar findings have been observed in humans, although diazepam’s effects have been less consistent. The dosage of GABAergic drugs is a critical factor in SDL induction. Contrary to initial assumptions of high-dose requirements, Colpaert demonstrated that even low, therapeutic doses of benzodiazepines like chlordiazepoxide can induce SDL, and recall doses can be significantly lower than encoding doses. This contrasts with early pentobarbital observations where optimal recall occurred at the same dose used during learning, and the amnestic barrier strengthened with dose deviations at testing. This dose-response variability may explain some inconsistencies in SDL research and underscores the importance of meticulous dose consideration for specific drugs and learning tasks.
Studies investigating the cross-substitution of GABAA receptor agonists in recovering state-dependent memories have revealed asymmetrical substitution patterns, suggesting distinct GABAA receptor subtypes mediate SDL. Ethanol, a less specific GABAA agonist, could recover memories encoded under diazepam or muscimol (more specific GABAA agonists), but diazepam or muscimol were ineffective in reversing ethanol-induced SDL. Similarly, amobarbital, binding to all GABAA subtypes, could restore memory, whereas diazepam, primarily targeting synaptic GABAA receptors, yielded inconsistent results. This points towards extrasynaptic, αβδ GABAA receptors potentially playing a crucial role in SDL. Unlike most γ subunit-containing GABAA receptors, αβδ receptors exhibit low benzodiazepine sensitivity but high sensitivity to low alcohol concentrations and gaboxadol. These extrasynaptic GABAA receptors regulate tonic inhibition and mediate the sedative, hypnotic, and anxiolytic effects of neuroactive steroids in mice. Our research has shown that gaboxadol strongly supports SDL, even in contextual fear conditioning, a paradigm typically resistant to state-dependent effects.
Recent bioinformatic analyses have revealed that microRNAs (miRNAs) also regulate GABAA receptors, in addition to various endogenous and exogenous agents. miRNAs fine-tune protein levels by degrading or blocking the translation of target mRNAs. Although individual miRNA effects on protein levels are subtle, their collective physiological impact is significant due to the simultaneous regulation of multiple functionally related proteins. We have discovered that miR-33, targeting several GABA-related proteins, strongly influences gaboxadol’s SDL-inducing capacity. Unlike miRNAs directly regulating learning and memory, miR-33 increases the threshold for gaboxadol’s actions, shifting the dose-response curve. Intriguingly, levels of several extrasynaptic GABAA receptors and GABAA-targeting miRNAs, including miR-33, are consistently dysregulated in patients with major psychiatric disorders like depression and schizophrenia. Whether these molecular abnormalities contribute to state-dependent information processing characteristic of these disorders remains an important question.
In summary, multiple neurotransmitter systems, particularly the GABAergic system, support SDL, leading to the formation of memories that are not easily accessible under normal conditions. Further research is needed to fully understand the mechanisms and significance of memory formation in different states. A key question is whether GABAergic mechanisms directly mediate SDL or if they induce states that alter the interpretation of ongoing glutamatergic transmission.
Cellular and Circuit Mechanisms Underlying SDL
Beyond GABAergic drugs, GABA receptors also mediate SDL induced by other substances like morphine. This is expected, as µ-opioid receptors are mainly expressed on interneurons, suggesting GABA receptors as downstream effectors of interneuron-related receptor mechanisms. Interneuron innervation of pyramidal excitatory neurons is domain-specific, enabling the coordination of glutamatergic inputs across different pyramidal cell compartments. This coordination is achieved through temporally distinct firing patterns of GABAergic interneurons, which modulate their activity during different network states. While interneuron-specific firing has primarily been linked to segregating cell assemblies and establishing temporal order during behaviors, it likely also contributes to SDL.
Early SDL studies indicated that different brain regions contribute differently to SDL. For instance, low-intensity electrical stimulation of the caudate nucleus and hippocampus is sufficient for SDL, whereas the amygdala requires strong stimulation inducing overt seizures to produce SDL effects.
Girden and Culler proposed that curare-induced conditioning is subcortical and either doesn’t require or is suppressed by cortical activity. Girden’s subsequent study showed that auditory cortex ablation in dogs eliminated dissociation between curare-induced and non-drug states. However, this was not replicated by Bliss, Sledjeski, and Leiman, who found intact SDL in monkeys with frontal ablations under pentobarbital. Robust circuit effects were demonstrated by lateralized state-dependency in split-brain rats, absent in intact rats. Our lab investigated the extended hippocampal circuit’s role in gaboxadol-induced SDL. Contextual fear conditioning normally depends on the hippocampus and retrosplenial cortex, but SDL under gaboxadol was independent of this cortical area and even enhanced by retrosplenial cortical inactivation. Analysis of suppressed cortical and elevated subcortical immediate early gene activation supports this view, suggesting that state changes involve altered routing of neuronal signals within broader brain circuits.
Brain states supporting learning are often defined by rhythmic neuronal activity at various frequencies. Many SDL-inducing drugs also alter electroencephalogram (EEG) patterns, as first observed with phentobarbital. Sadowski and Longo found that EEG synchronization after scopolamine administration mirrored the disruption of responses learned in a non-drug state. Leiman, Bliss, Powers, and Rosenzweig showed that pentobarbital, at dissociative doses, shifted rat EEG activity from low-voltage desynchronized (arousal) to high-amplitude synchronized waves. It’s now well-established that SDL-supporting drugs, including gaboxadol, scopolamine, and opiates, induce changes in oscillatory neuronal activity measured by EEG or local field potentials. These significant shifts in electrical activity likely correlate with behavioral drug dissociation. Thus, molecular events like extrasynaptic GABAA receptor activation can alter local and global network activity, enabling state-dependent memory encoding and retrieval (Figure 2).
Figure 2. Molecular, Cellular, and Circuit Mechanisms of State Dependent Learning.
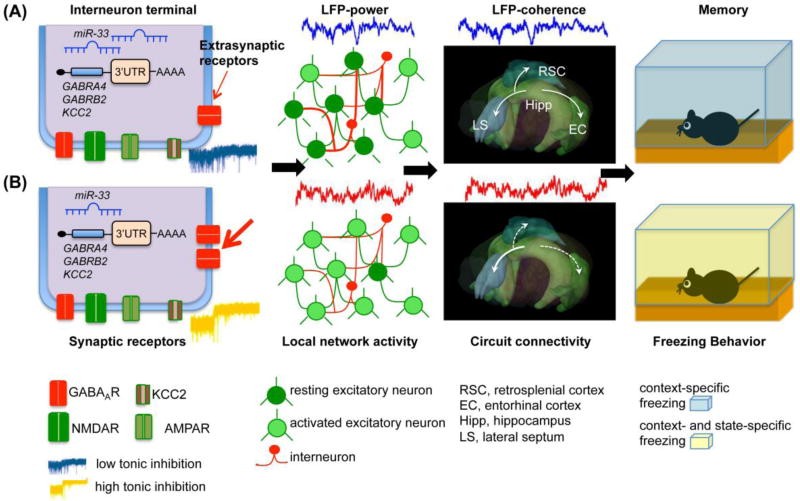 Model of SDL based on extrasynaptic GABAAR activation on hippocampal dentate gyrus interneurons.
Model of SDL based on extrasynaptic GABAAR activation on hippocampal dentate gyrus interneurons.
Alt Text: Illustrative model depicting the molecular, cellular, and circuit mechanisms of State Dependent Learning (SDL), focusing on the role of extrasynaptic GABAA receptors on hippocampal dentate gyrus interneurons.
(A) Normal Tonic Inhibition and Memory Retrieval: This panel represents conditions with normal tonic inhibition (indicated by a thin red arrow). In this state, some excitatory granule cells are activated, inducing changes in local network activity and coherent activity between the hippocampus and its cortical and subcortical targets. These changes correlate with successful memory retrieval in contextual fear conditioning, manifested as freezing behavior during memory testing. (B) Increased Tonic Inhibition and Disrupted Memory Retrieval: This panel depicts increased tonic inhibition mediated by extrasynaptic GABAA receptors (thick red arrow) on interneurons. This increased inhibition paradoxically leads to disinhibition of granule cells, increasing the number of active cells. This state induces changes in local and global oscillatory activities, resulting in disrupted hippocampal-cortical processing and enhanced hippocampal-subcortical processing of context memories. Memories encoded in this state are best retrieved when extrasynaptic GABAA receptors are reactivated, recreating the encoding state. KCC2 (potassium chloride cotransporter 2) is also indicated in the diagram.
Conclusion and Implications of State Dependent Learning
Despite its fundamental nature, the phenomenon of SDL has received limited attention recently. This is surprising given the significant insights a deeper understanding of SDL’s neurobiology could offer. These insights span: (i) fundamental principles of information processing, (ii) the behavioral impact of inaccessible memories, (iii) SDL’s role in the development and maintenance of psychopathology, and (iv) information processing in psychiatric conditions.
While drug-induced SDL is frequently studied in research, it may be a common aspect of everyday information processing. Emerging research suggests that spatiotemporal patterns of spontaneous neuronal network activity serve as memories, and state-dependency may explain the diversity of these patterns. From an evolutionary perspective, SDL can be viewed as a mechanism for organizing memories to influence decision-making specifically when accessing certain information is advantageous. SDL is also considered a protective mechanism, temporarily shielding individuals from negative emotions associated with distressing memories.
However, relying heavily on SDL as a learning strategy can have adverse consequences. Incompletely integrated memories and emotions during encoding can increase vulnerability to psychiatric disorders, particularly dissociative disorders and post-traumatic stress disorder (PTSD). State-dependent traumatic memories, though not fully retrievable, can significantly influence social and affective behavior. For example, emotion processing, a crucial aspect of social cognition, is state-dependent in schizophrenia patients. SDL is also implicated in drug addiction persistence, as drug use can become a strategy to access drug-state-encoded information. This potential role of SDL is particularly relevant given the widespread and increasing recreational and prescription drug use and abuse. Ultimately, understanding SDL mechanisms can enhance our comprehension of psychiatric state phenomenology and psychotropic drug actions. By facilitating information transfer across different states, we may develop more effective treatments for various psychiatric and neurological disorders.
Highlights
- SDL can be triggered by a wide array of endogenous and exogenous stimuli.
- Many drugs inducing SDL converge on GABAergic neurotransmission.
- GABAergic induction of SDL is modulated by microRNAs.
- SDL involves alterations in circuit and global network activity.
- SDL is a fundamental learning mechanism and a potential pathway to psychopathology.
Acknowledgments
Research in the authors’ laboratory was supported by grants MH078064 and MH108837 from the National Institutes of Mental Health to J.R and the Neurobiology of Information Storage Training Grant MH067564 to M.A.A.M.
Footnotes
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
